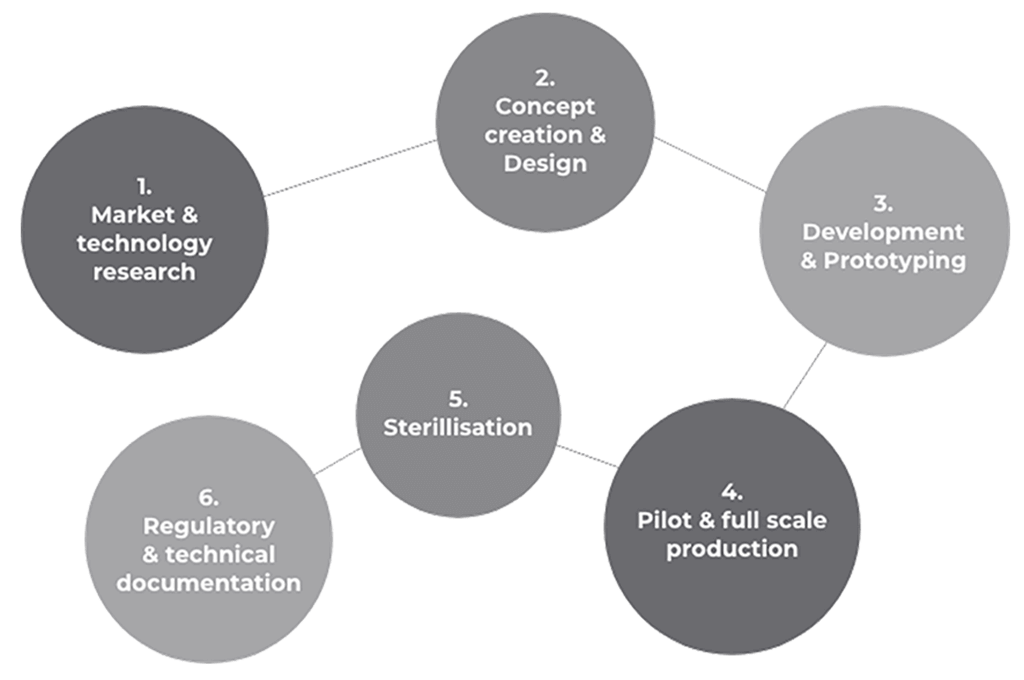
Have you had a bright idea? We are the experts in designing, developing and manufacturing end-to-end advanced medical and surgical solutions - providing specialist knowledge in thermoplastic engineering, tubing systems, and regulatory compliance.
We take your product ideas from consultation to concept to production, and work alongside you to bring medical innovations to life.